On the periodic table the majority of elements are metals and some of elements are non metals.
At room temperature most of the elements in the periodic table are.
Francium symbol fr and atomic number 87 a radioactive and reactive metal melts around 300 k.
Liquid at room temperature.
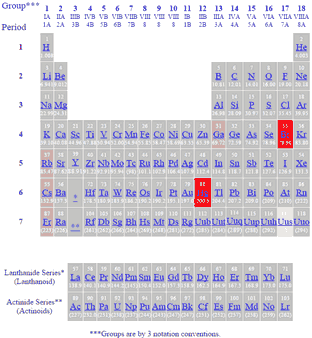
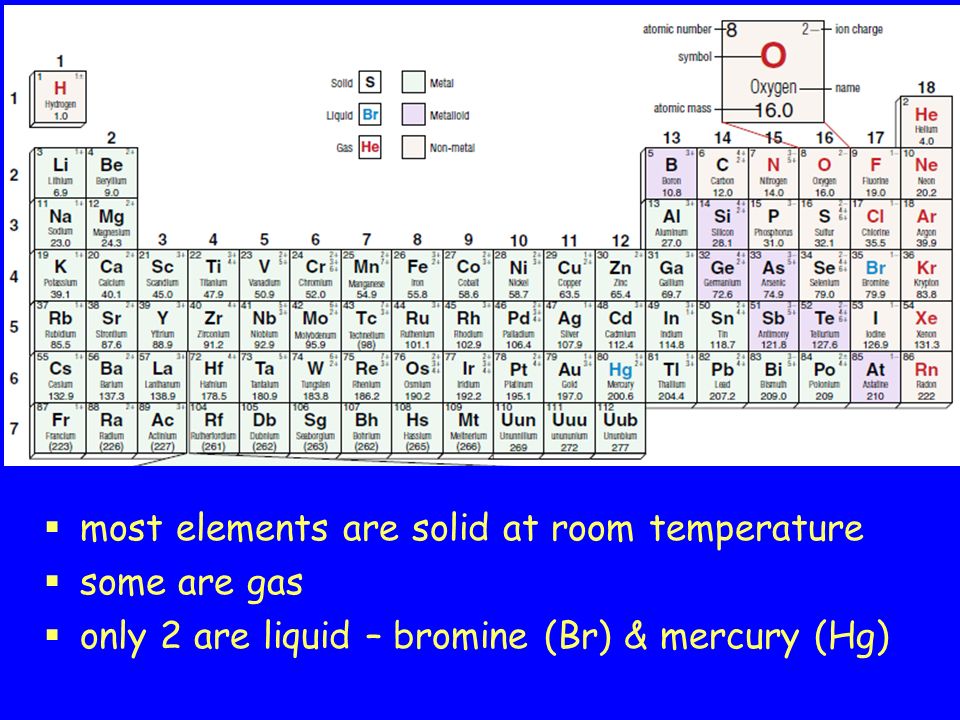
The only liquid elements at room temperature on the periodic table are mercury and bromine check the related link for a dynamic periodic table which shows the state of each element at various.
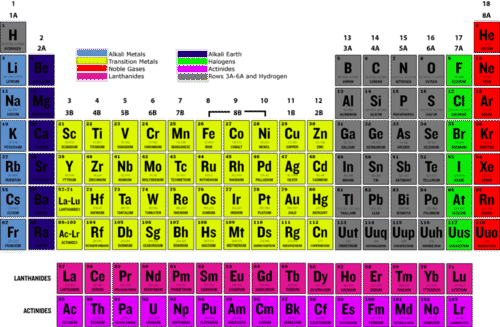
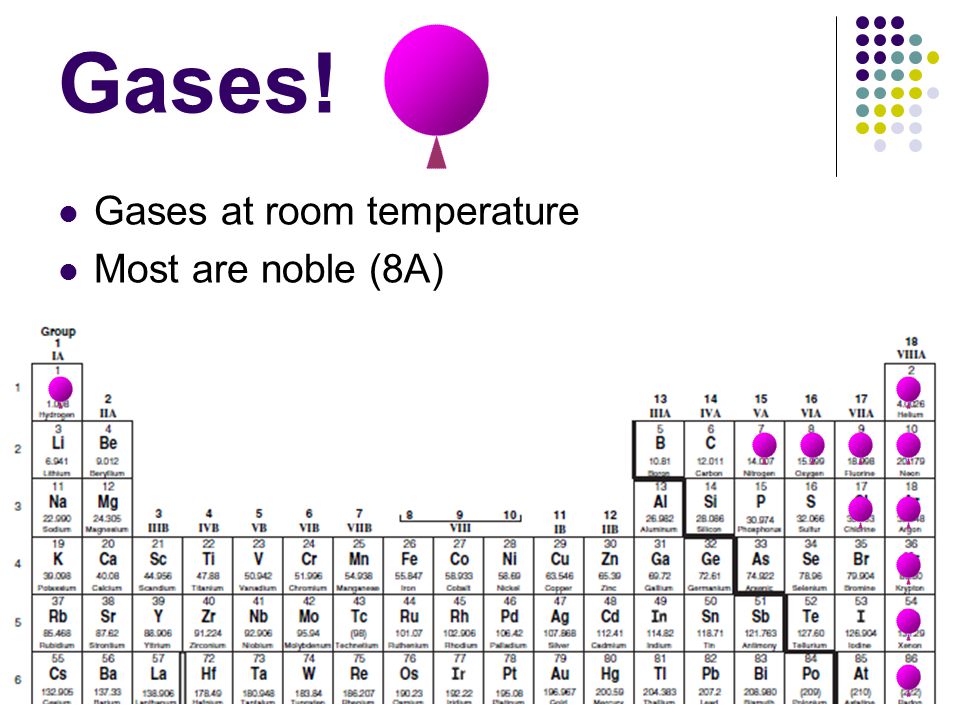
And include fluorine chlorine bromine iodine and astatine.
Radon helium xenon neon krypton and argon are eight noble gases.
Elements of the periodic table post a photo of your element in its pure form.
Mercury hg and bromine br are the only elements in the periodic table that are liquids at room temperature.
They are nonreactive mono atomic elements with extremely low boiling points.
If you cannot do that describe its pure form and post a photo relating to it with a description how it is related to your element.
Most metals are not.
At room temperature more than half of the nonmetal elements are.
Solid at room temperature 84 these elements are solids at room temperature and pressure.
They are the only group whose elements at room temperature include solid liquid and gas forms of matter.
Most of the metals are solids under ordinary conditions i e 25ºc 1 atmosphere of pressure etc with the exception of mercury hg element 80 which solidifies.
Which category of elements contains the most elements.
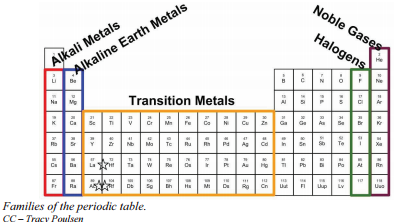
Halogens are the non metallic elements found in group 17 of the periodic table.
Click any element below to see all the samples of that element.
It is solid at room temperature but it ignites when heated above 150 c or higher in moist air it tarnishes to the oxide.
It is a.
In an atom the number of protons equals the number of.
Photographs and descriptions of many samples from the collection solid at room temperature in the periodic table.
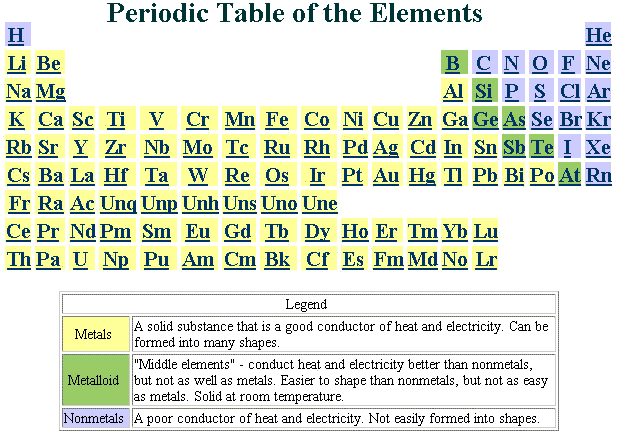
The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties the structure of the table shows periodic trends the seven rows of the table called periods generally have metals on the left and nonmetals on the right.
Francium is the most electropositive of all the elements.
Most metals have a high melting point which means therefore the answer to this question is solid.
Each of the 13 elements has their own unique physical and chemical properties.